Antibody-Drug Conjugates (ADCs) offer a powerful approach to cancer therapy by combining monoclonal antibodies with cytotoxic drugs for targeted treatment. This precision reduces harm to healthy cells while maximizing therapeutic impact. By the end of 2022, 14 ADCs had received FDA approval, with over 100 in clinical trials. However, their complex structures and unpredictable biotransformation require sophisticated analytical tools. Understanding ADC behavior through adc analysis and bioanalysis—especially pharmacokinetic (PK) studies—is essential. This article highlights LC-MS-based strategies, featuring WuXi AppTec DMPK’s expertise in overcoming challenges and advancing ADC development.
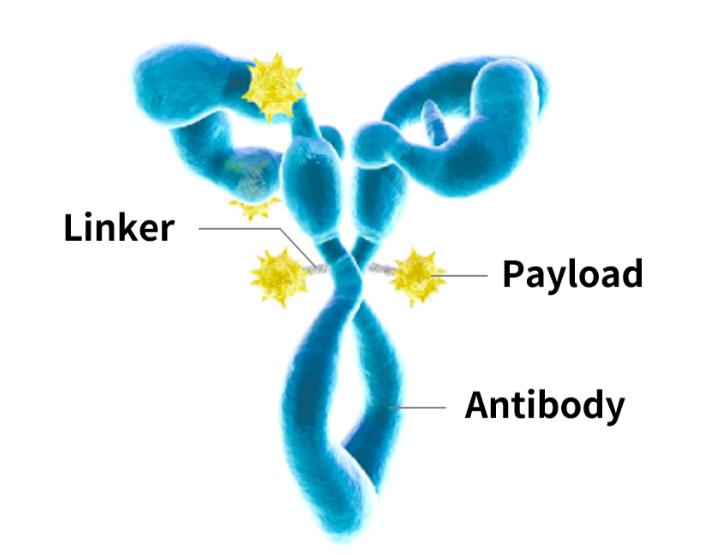
Key Bioanalytical Techniques in ADC Development
LC-MS (Liquid Chromatography-Mass Spectrometry) in ADC Bioanalysis
LC-MS has emerged as a cornerstone of ADC bioanalysis due to its speed, precision, and ability to handle complex samples. It enables researchers to quantify various ADC components including total antibodies, conjugated payloads, and unconjugated drugs. LC-MS can assess pharmacokinetics, evaluate drug-to-antibody ratios (DAR), and monitor biotransformation. At WuXi AppTec, advanced LC-HRMS platforms are used to obtain high-resolution data and accurate molecular profiling.
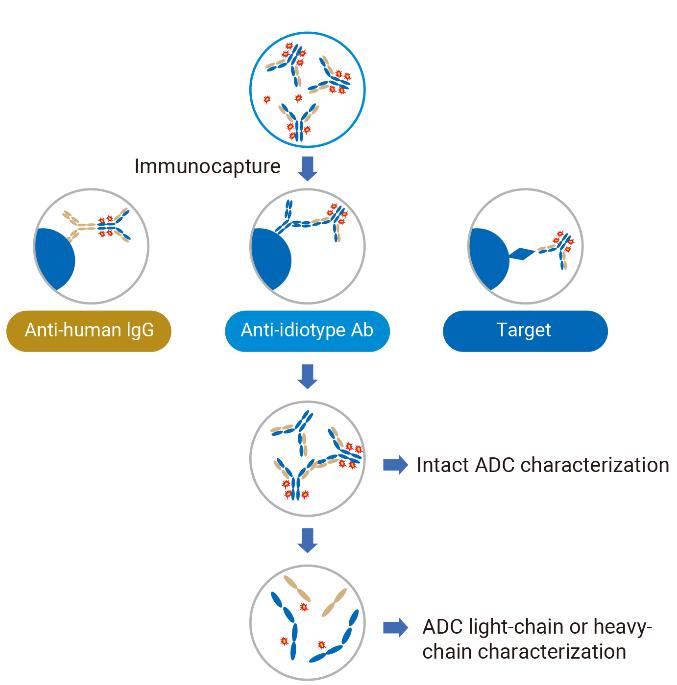
The use of LC-MS also allows for detailed DAR distribution analysis, revealing the proportion of ADC molecules with varying drug loads. With immuno-affinity capture and direct LC-MS analysis, even subtle structural changes in ADCs can be detected. These capabilities streamline the development process and reduce reliance on slower methods like ELISA.
Other Bioanalytical Methods in ADC Development
While LC-MS is dominant, other techniques still play roles in specific contexts. Ligand Binding Assays (LBAs) such as ELISA remain standard for total antibody quantification, especially during early-stage development. LBAs can also detect anti-therapeutic antibodies (ATA). High-Resolution Mass Spectrometry (HRMS), when integrated with affinity capture techniques, provides precise structural insights. Each of these tools complements LC-MS, offering a broader analytical scope for accurate and reliable ADC bioanalysis.
Challenges in ADC Bioanalysis and How to Overcome Them
Drug-to-Antibody Ratio (DAR) Determination Challenges
DAR is a critical parameter in ADC design, directly affecting efficacy and safety. Most ADCs today are heterogeneous, with DARs ranging widely across molecules. An average DAR of 3 to 4 is considered ideal; higher values can cause toxicity and faster clearance, while lower values may reduce therapeutic effect.
To address this, WuXi AppTec developed a robust LC-HRMS method for intact protein analysis. Using immuno-affinity capture followed by high-resolution mass spectrometry, researchers can identify specific DAR peaks, even within complex biological matrices. Deconvolution software then processes this data to determine accurate DAR distributions, significantly improving reliability over traditional HIC-UV methods.
Analyzing Payload Release and Metabolites
Understanding how and when the cytotoxic payload is released is essential for evaluating ADC safety and effectiveness. Payloads released prematurely can harm healthy tissue; those released too late may miss their therapeutic window.
Advanced LC-MS methods enable accurate quantification of both conjugated and free payloads. These analyses are crucial during in vivo studies to trace how ADCs transform after administration. WuXi AppTec's platform allows for quantification of standard payloads like MMAE and DM1 across a wide dynamic range, even at low concentrations. This ensures high-confidence data for PK and toxicology evaluations.
Advancements in ADC Payload and Linker Technology
Cleavable vs. Non-Cleavable Linkers: Impact on ADC Performance
Linkers are the molecular bridges that attach payloads to antibodies. Cleavable linkers release the drug inside the target cell, triggered by pH, enzymes, or other intracellular conditions. They are ideal for ensuring drug release only in tumor environments, enhancing selectivity.
Non-cleavable linkers, on the other hand, rely on the degradation of the antibody to release the drug. This can improve stability in circulation but may reduce the rate of payload delivery. Bioanalytical evaluation is essential to determine which linker type suits specific clinical goals.
WuXi AppTec employs LC-MS and affinity capture techniques to assess linker performance. These analyses confirm whether payload release occurs as intended, preventing off-target toxicity.
Emerging Payload Technologies and Their Bioanalytical Implications
Next-generation ADCs are incorporating novel payloads beyond traditional cytotoxins. These include DNA-damaging agents, immunomodulators, and tubulin inhibitors with increased potency and specificity.
As these payloads become more diverse, their bioanalysis becomes more complex. Some may require unique extraction methods or more sensitive detection platforms. WuXi AppTec’s team uses both standard (e.g., protein precipitation) and advanced methods (e.g., solid-phase extraction) to optimize quantification.
Mass spectrometry remains central to evaluating new payloads. Techniques like LC-MS/MS are adapted to handle new chemical structures, ensuring robust characterization. These innovations enable more flexible and targeted therapy designs, paving the way for safer, more effective ADCs.
The Future of ADC Bioanalysis: Innovations and Trends
AI and Automation in ADC Bioanalysis
Automation and artificial intelligence (AI) are transforming bioanalytical workflows. Automation reduces manual errors and increases throughput, particularly in sample preparation and data handling.
WuXi AppTec integrates automated platforms to streamline LC-MS-based ADC analysis. Automated sample handling improves consistency, while AI algorithms accelerate data interpretation. Machine learning can identify patterns in DAR distribution or payload metabolism, offering new insights faster than traditional analysis.
These tools help shorten the development cycle and improve data reliability, making ADC programs more efficient and scalable.
Personalized Medicine and Its Role in ADC Development
The shift toward personalized medicine is influencing ADC development strategies. Different patients may metabolize ADCs differently based on their genetic profiles, tumor microenvironment, or immune response.
To support personalized approaches, bioanalytical methods must be flexible and precise. This includes the ability to detect patient-specific biomarkers or monitor ADC metabolism in individual subjects. LC-MS offers the adaptability needed to tailor testing protocols based on patient needs.
WuXi AppTec’s customizable bioanalysis services can adapt LC-MS methods for specific patient populations or tumor types. This facilitates the design of more effective, individualized therapies, ensuring better clinical outcomes.
Conclusion
Antibody-Drug Conjugates (ADCs) mark a major advancement in targeted cancer therapy, but their complexity requires robust and precise bioanalytical strategies. Techniques like LC-MS are essential for analyzing DAR, monitoring payload release, and understanding pharmacokinetics. WuXi AppTec offers an integrated ADC analysis platform with advanced LC-MS, expert interpretation, and adaptable protocols. As linker and payload technologies advance, and with the rise of automation and personalized medicine, bioanalysis becomes increasingly vital. These strategies help researchers improve therapeutic precision, reduce risks, and accelerate ADC development, paving the way for more effective cancer treatments.
